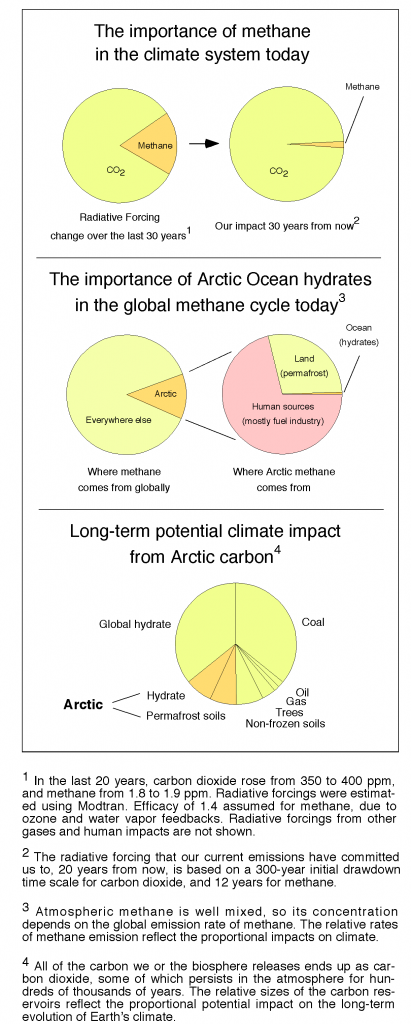
The thing that really gets me in the gut about global warming from fossil fuel combustion is how long it will last. Carbon mined from the deep Earth and injected into the “fast carbon cycle” of the atmosphere, ocean, and land surface will continue to affect atmospheric CO2 concentrations, and climate, for hundreds of thousands of years into the future, unless we clean up the atmosphere ourselves.
It turns out that human emissions of the element mercury (Hg) will elevate mercury concentrations in the environment, and in upper trophic-level seafood, for thousands of years into the future. There are a lot of parallels to the carbon cycle. But, unlike the carbon cycle, the mercury cycle would be impossible to clean up.
[Read more…] about Mercury, the other geologically persistent planetary poison