
Guest Commentary by Drew Shindell (NASA GISS)
The current winter and early spring have been extremely cold in the Arctic stratosphere, leading to the potential for substantial ozone depletion there. This has been alluded to recently in the press (Sitnews, Seattle Post Intelligencer), but what’s the likely outcome, and why is it happening?
First, let’s go over some background. The recipe for massive springtime ozone loss in the polar regions, such as the annual ozone hole seen over Antarctica during the past two decades, is fairly simple. The two main ingredients are reactive halogen gases such as chlorine or bromine and sunlight. To prepare, keep the halogens at extremely cold temperatures, typically below –78 C (195 K). Use a strong polar vortex to mix the halogens to help achieve the required temperatures. When the mixture has been properly chilled, add sunlight and you’ll get rapid ozone destruction.
In the real world, both chlorine and bromine are readily available in the stratosphere worldwide, especially chlorine from chlorofluorocarbons which are well mixed in the lower atmosphere (where they are stable), before entering the stratosphere where they are photochemically decomposed. The halogens that are released generally end up in fairly unreactive forms where they have only a small effect on ozone. Under extremely cold conditions, however, ice and supercooled liquid droplets (so-called Polar Stratospheric Clouds – PSCs) can form even at the low densities present in the lower stratosphere (~15-25 km altitude). Chemical reactions on the surfaces of these particles can rapidly convert halogens into very reactive forms. Ozone depletion is therefore extremely sensitive to small changes in temperature when the stratosphere is near this freezing point. The temperatures themselves are greatly influenced by the strength of the polar vortex, a wind that swirls around the pole and when strong, can keep air confined throughout the winter in the polar night, allowing it to cool dramatically. The primary reason ozone depletion has been weaker over the Arctic than over Antarctica is than Arctic temperatures are typically about 10 degrees warmer as the Arctic vortex is generally weaker than its Antarctic counterpart. This is because of the differences in layout of the continents in the two hemispheres affects the dynamics of stratospheric circulation.
The other key factor is that even if chemical conversion into reactive forms occurs during the cold, dark polar winter, the reactive chlorine must stick around until sunlight returns to the polar region for ozone destruction to take place. This is why ozone depletion over the poles is a springtime phenomenon. Even following a very cold winter, if temperatures warm quickly during spring very little ozone loss may take place. Alternatively, a milder winter, provided it was still cold enough to lead to chemical processing of halogens, could be followed by greater springtime ozone losses if temperatures stayed cold longer. Thus temperatures during the period when a lot of sunlight first returns to the polar areas following winter, March in the Arctic and September in the Antarctic, are crucial.
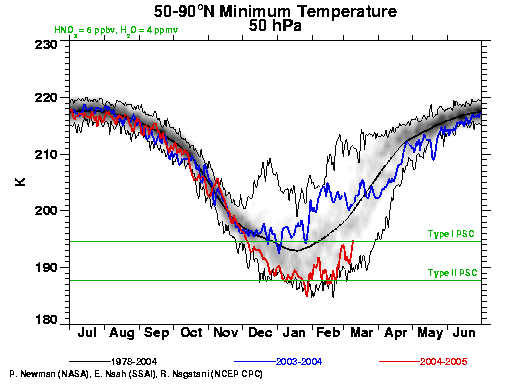
This year has seen an exceptionally strong polar vortex over the Arctic (see the red line in the figure). Undoubtedly, chemical processing of halogens into reactive forms has taken place and the Arctic is primed for ozone depletion. Now that we’re in March, sufficient sunlight is available to cause sizeable ozone losses. Should cold temperature persist for another couple weeks, ozone depletion could reach record levels for the Arctic. While the vortex was weakened and pushed to the side of the Arctic during the last week of February, temperatures below the critical freezing point are still present as of March 9 (see figure). The displacement off the pole also pushes the colder air into latitudes with more sunlight, enhancing ozone depletion in the short term. Ozone measurements from the first week of March already show a region over the North Atlantic with very low ozone levels (<250 Dobson units, versus minimum values of ~300 in the early 1980s).
There is much debate over whether this has anything to do with climate change. Some climate models suggest that increasing greenhouse gases may be leading to a gradual strengthening of the Arctic vortex and hence increasing ozone losses, while others do not. Observations show that the vortex was typically more stable in the 1990s than during the 1980s, but the present decade has been mixed thus far. Temperature during the winter as a whole have generally decreased over the past two decades, likely as a result of climate change, but the sensitivity of ozone loss to the exact timing of March warming events makes ozone depletion a much more variable quantity. With only one winter vortex per year, it will take many more years to determine if the exceptionally cold 2004-2005 winter is part of a trend or simply a single cold event. Prepare to see a lot of press coverage if this ends up being a big year though….
Update: Indeed it was.
How does this relate generally to stratospheric cooling over recent decades and the apparent positive feedback whereby ozone loss causes further cooling which leads to further ozone loss… Here’s a good overview news feature Ozone And Climate Change from the Earth Observatory at NASA.
What of the connection with solar storms? Isn’t this what some researchers are now saying was responsible for the sudden drop in ozone? This phenomenon also has some people calling for more study on the connection between climate change and activity taking place outside of the Earth’s atmosphere. Has there been much research in this area? I don’t know if I am confusing the issue here between ozone and climate change.
[Response:The ‘solar storms’ story was about the ozone loss last winter (i.e. 2003/2004) where the coincidence of a severe solar storm and the normal spring time depletion gave rise to a particularly severe loss. However, you can see from the graphic (blue line) that the vortex last year was nowhere near as cold as it has been this winter. Solar storms of this severity are rare, and so don’t come in to the picture very much on a climatic basis. – gavin]
Now that we’re on ozone depletion, I read a few years ago that NOx over mid-latitudes in N. America, due to agriculture (chem fertilizers) & car-driving, was causing a thinning of the ozone layer. Is that right?
Are developing economies (i.e. China/India et.al.) still producing the very CFC’s that were phased out in developed countries when the Montreal Protocol was implemented? I am just wondering where after all the bad stuff is coming from, and whether the Montreal Protocol is working as planned or not. Thanks, for a great topic. Peace.
D
I read about this in January, when many papers cited Markus Rex from PIK on that problem: the science behind this scare story seems correct, but the real data are stubborn: Rex predicted a severe loss for January (maybe February), and the data showed none: look here http://www.temis.nl/protocols/o3field/data/forecast/today_np.gif
for daily data (the forecast is based on prior measurements).
Now the time of depletion slips to spring… and I am still waiting to read that back in January/February there was no major problem! I doubt that even a temporary (as it might happen) depletion will impose a greater risk for UVB damage on population than spending a single session in the sun-tan studio. So please, let’s continue to look at the measurements, and cry “wolf” only when danger is there!
It’s important not to be short term sighted on global warming and depletion of the ozone layer.
With global warming just beginning, heat waves are rare now; but the frequency and severity of heat waves will increase with time.
With ozone layer depletion just beginning, large holes in the ozone layer are rare now; but the frequency and severity of ozone layer depletion will increase with time.
Big questions
How fast will the global climate warm, and how fast will the ozone layer be depleted?
RE #4
This post is a great post. I have seen media stories about the recent northern hemisphere ozone deletion and this post has answered alot of my questions.
Dusty Bradshaw poses a good question. It made me curious, and I have a background in environmental regulation so I took a few minutes to do some research.
The United Nations Environmental Program has some great information on this. The Ozone Secretariat in the UNEP deals with the Montreal Protocol. According to the Secretariat CFC production has dropped considerably. This information is at:
http://www.unep.org/ozone/index.asp
There is a ton of facts on this site. You can look up which countries have ratified the Montreal Protocol:
http://www.unep.org/ozone/Treaties_and_Ratification/2C_ratification.asp
The Frequently Asked Questions has some great publications that address scientific questions, environmental effects and the impact of the Montreal Protocol:
http://www.unep.org/ozone/Public_Information/4D_PublicInfo_FAQ.asp
In Earth’s past, what was the ozone layer like during the globally high temperature episodes (55 mya, 100 mya, 220 mya)? Major extictions took place during these episodes. The extinctions near the late Paleocene Eocene Thermal Maximum (PETM) 55 mya were mainly marine species. It seems likely that depletion of the ozone layer near the PETM led to a loss of plankton and resulting collapse of marine ecosystems. Evidence exists of ultra violet radiation in late Permian extictions 230-250 mya. I haven’t read anything on what may have contributed to the extinctions during the Cenomanian Turonian (94-100 mya). It seems likely that ozone depletion contributed to the major extinctions that took place during these warm global periods.
Major extictions also occurred at the end of the Cretaceous 65 mya, believed to be related to an asteroid crash into the sea off the Yucatan Peninsula. Is it possible that the ozone layer was depleted from water vapor that entered the stratosphere from the big splash?
[Response: The problem with hypotheising ozone changes in the past is that ozone leaves no unique geochemical trace, and thus you have to rely on models completely to fill in the gaps. However, ozone reponses to climate changes are quite sensitive to the initial base state (how much stratospheric water vapour was there? how much methane? volcanic aerosols? etc.) since it forms as a delicate balance between UV-related production and chemical loss (which is highly non-linear). I would be very hesitant in attributing mass extinctions to ozone losses. In the PETM case, we did some calculations showing around 20% max depletion for various CH4 release scenarios. I’m sure someone will do a better calculation at some point, but this first cut doesn’t seem high enough to justify your claim. Global warming of around 4 to 5 degrees is a more likely cause. – gavin]
I wrote:
>> It seems likely that depletion of the ozone layer near the PETM led to a loss of plankton and resulting collapse of marine ecosystems.
gavin replied:
> I would be very hesitant in attributing mass extinctions to ozone losses.
My comment:
It seems unlikely to me that a 4 to 5 degree warming of the atmosphere would have caused the PETM marine extinctions. I think the food supply (plankton) for marine ecosystems was cut off near the PETM. What happened to the PETM marine vegetation and why?
How important is the ozone layer in protecting vegetation on water, and land? Vegetation is at the bottom of the food chain. If vegetation dies, …
This may be of interest:
Ozone values over the UK are currently quite low. This afternoon I measured 230 DU over BAS, in agreement with the forecast. Values are normally around 350 DU at this time of year. Values are probably at their lowest today, but will remain significantly below average over the weekend.
Which i got from Jonathon Shanklin in Cambridge.
Answer to #10:
Ozone values are ***VERY*** variable: even if there is a mean sinusoidal pattern over Europes latitudes ( see http://www.meteo.be/english/pages/OzonEN.html or http://meteo.lcd.lu/dobson05.html ) the variations from that mean trend are exceptional great: as such a local measurement of low values is never a hint to a lowering trend (and the opposite is true also…) .
Btw, I am still waiting for ( Gavins ?) Arctic ozone loss happening… read my comment #5 and again watch: http://www.temis.nl/protocols/o3field/data/forecast/today_np.gif
for a very healthy O3 layer ….
Data from the Swedish Odin satellite indicate that no arctic ozone hole will appear this winter, despite fears to that effect.
See for the full article:
http://www.spacedaily.com/news/ozone-05d.html
Comment on #5 and #11:
The Arctic ozone layer is very variable mainly for dynamical reasons. This makes it difficult to separate chemical ozone loss from natural changes induced by transport. Sophisticated approaches are needed to reliably quantify anthropogenic chemical loss of ozone from observations. A number of approaches have been developed over the past couple of decades and these are well documented in the literature. But it is also well known that just looking at fields of total ozone does not tell us much about chemical loss (total ozone is a measure of the “thickness” of the ozone layer). The link given in the comment of Francis Massen points to a total ozone map. The comment suggests that by looking at this map everyone can convince himself that no chemical ozone loss took place so far. But total ozone maps are not able to support any statement about chemical ozone loss in the Arctic. Due to transport processes total ozone over the Arctic and northern mid-latitudes increases each winter. Depending on the meteorological conditions during the winter, chemical loss chews away part of this increase. The transport processes are also very variable from winter to winter and the amount of ozone pumped into the Arctic is also correlated with temperature (this is not a causal relationship – the correlation exists, because variability in temperatures and in ozone transport are both driven by the same atmospheric processes). From just looking at total ozone, how can we seperate natural variability in transport from anthropogenic chemical loss ? Just one example how problematic the interpretation of total ozone maps is. Look at:
ftp://toms.gsfc.nasa.gov/pub/eptoms/images/npole/Y2005/IM_oznpl_ept_20050319.png
Looks like a pronounced ozone hole over the UK. But the main reason for this situation was a natural transport phenomenon. Anthropogenic chemical loss also contributed to it, but was not the main cause for the unusually thin ozone layer above the UK in this situation. There is no way to tell this from just looking at the total ozone map.
We have developed an approach to quantify chemical loss of ozone and to seperate it from the natural variability induced by transport. The approach has been well documented in many articles in the peer-reviewed literature. During a recent meeting in Zuerich, where many of the European scientists that study Arctic ozone have been present, we have discussed the current situation in the Arctic. The common conclusion from a number of independent approaches (including ours) was that up to date more than 50% of the ozone at 19-20 km altitude have been chemically destroyed this winter. This is about as bad as it has ever been for Arctic ozone around this time of the year. So far the degree of Arctic ozone loss in winter 2004/2005 is very similar to the previous record loss in the Arctic, that occurred during winter 1999/2000. Unfortunately the concern we had in January/February turned out to be justified.
A stratospheric warming took place about two weeks ago and conditions for Polar Stratospheric Clouds were no longer present since about ten days. But the warming does not have an immediate effect on ozone loss and our observations show that ozone loss still continues these days. This is consistent with our understanding of the ozone loss process and model calculations. Very recently the final breakdown of the polar vortex started. But again this does not prevent further ozone loss, because stable remnants of the polar vortex often survive the breakdown for weeks and ozone loss can continue in these remnants. A very comparable situation led to the record loss in winter 1999/2000. Although the vortex broke down around mid-March of that winter, ozone loss continued in a stable remnant and by early April about 70% of the ozone at 20 km was destroyed in this fragment of the vortex.
But it is important to put the measured ozone losses at individual layers into some perspective: 19-20 km altitude is where the maximum ozone concentration is normally reached over the Arctic. So the maximum loss occurrs in the heart of the ozone layer. But still, above and below this altitutde region chemical loss of ozone was so far always much more limited. E.g. in 1999/2000 about 30% of the total column ozone was destroyed by anthropogenic chemical loss, which is considerably less than the maximum loss at 19-20 km altitude. Also, the overall number of ozone molecules destroyed in a vertical column of air was pretty much the same as the number of molecules transported into this column by the average poleward and downward transport of air in the stratosphere. Hence, the chemical loss has been balanced by transport, such that in spring 1999/2000 the ozone layer above the Arctic has still been as thin as it always is in fall, in contrast to the normal seasonal increase of the thickness of the ozone layer during winter. So UV-levels in spring have been higher than in “normal” winters. But they have not been higher than during summer or even in fall. So it is important not to overstate the problem. On the other hand the depletion of the ozone layer in spring certainly can have a significant effect on ecosystems, although so far the ozone layer has not been thinner in spring than later during the year. Plants and animals have adapted to the normal seasonal cycle of total ozone and UV and are not used to high UV-exposure during the part of their lifecycle that takes place in spring (e.g. germination, growths of buds, algea blooms in the Arctic ocean, … ). To my knowledge (which is quite limited in this aera) the effect of increased UV-levels during spring on the various ecosystems is subject to ongoing reserach.
Comment on #2:
The paper by Randall et al. (which is the basis for the comment by Nigel Allan) shows a 60% depletion of ozone *at around 40 km altitude*. This is well above most of the ozone layer. Ozone depletion up there does not have any noticable effect on the thickness of the ozone layer. The bulk of the ozone is at lower levels and is not affected by the processes decribed by Randall et al.. The effect is scientifically very interesting, but it is not related to any signifiant thinning of the ozone layer. Any connection with the ozone hole that has been made in the media is nonsense. Unfortunately this story led to considerable confusion in the public. 2003/2004 was a relatively warm winter in the Arctic stratosphere and significant loss of ozone did not occurr. The effect of solar activity in fall 2003 was strong, but it was limited to levels well above the actual ozone layer.
Comment on 13:
I fully agree with Markus Rex that total ozone measurements can not give any clues on chemical destruction in isolated layers, and that we may have been lucky that O3 transport cancelled out the chemical thinning that happened during the last months. But what remains is that UVB levels at ground are a function of the total ozone column, and as that column remains very high, there is no need to panic on an hypothetical but inexistant UVB increase. What are the chances that the helpful O3 transport into the Artic would stop for an extended period? I would really be interested in seing actual arctic ground UVB measurements made during the last and next months…maybe Markus knows of a link to these data?
PS: do not forget that UVB measurements are rarely more accurate than 3 to 5% !