
Guest post by Bart Verheggen, Department of Air Quality and Climate Change , Energy research Institute of the Netherlands (ECN)
The impacts of aerosols on climate are significant, but also very uncertain. There are several reasons for this, one of which is the uncertainty in how and how fast they are formed in the atmosphere by nucleation. Here, in part I, I’ll review some of the basic processes that are important in determining the climate effects of aerosols, focusing in particular on their formation. This is also relevant in order to better understand –and hopefully quantify- the hypothetical climate effects of galactic cosmic rays which I’ll discuss in a follow-up post.
Background
Aerosols are liquid or solid particles suspended in the atmosphere (but not including water droplets or ice crystals). They can either be directly emitted into the atmosphere (primary aerosols like dust), or they can be formed in the atmosphere by condensation (secondary aerosol like sulfates). Almost all of their properties, and thus effects, are size dependent: The particle size governs the rate at which they fall out (and thus atmospheric lifetime), their interaction with radiation, their impact on clouds, or even their health effects. And they come in very different sizes, ranging from a few nanometers to tens of micrometers. Some sites with good introductory explanations to aerosols and their climate effects are here, here and here (German). RC also had some posts on the same generic topic here and here.
Climate effects of aerosols
Aerosol particles can influence climate in several ways: They scatter and absorb (in the case of black carbon) solar radiation (direct effects). They also act as cloud condensation nuclei (CCN) around which clouds can form, and thereby influence cloud reflectivity and cloud lifetime (indirect effects). Black carbon can have another indirect effect by changing the albedo of snow and ice, but that’s not the topic of this post. The aerosol indirect effects are the greatest source of uncertainty in assessing the human impact on climate change (reviewed here. The main idea is that more CCN causes liquid clouds to consist of more, but smaller, droplets. The resulting cloud is more reflective (first indirect effect). Due to the smaller size of cloud droplets, the formation of precipitation may be suppressed, resulting in a longer cloud lifetime and larger cloud cover (second indirect effect).
The mass of a freshly nucleated aerosol particle is more than 100,000 times smaller than that of an ‘aged’ aerosol of a size optimal to affect climate. As a rule of thumb, particles have to grow past 100 nm (1 nm = 10-9 meters) in order to become climatically active; below this size they are not easily activated into a cloud droplet and they don’t scatter solar radiation very efficiently. It is thus not immediately obvious that the climate effects of aerosols will depend very strongly on nucleation; the dependence is likely considerably damped, because a lot can happen to the aerosol particle as it comes of age.
Aerosol formation
The most prevalent trace gases do not generally nucleate new aerosols (or even condense onto existing ones), because they are too volatile (i.e. they have a high saturation vapor pressure and thus evaporate readily). They first have to be oxidized (usually under the influence of sunlight) to produce a compound with a lower vapor pressure. The prime example of this is the oxidation of sulfur dioxide (SO2) into sulfuric acid (H2SO4), which has a very low vapor pressure. The H2SO4 can then condense together with water vapor (and perhaps organic compounds and/or ammonia) to form a stable cluster of molecules: A new particle is typically 1-2 nanometers in diameter. Ions can also play a role, by lowering the energy barrier that needs to be overcome: The attractive forces between the molecules are stronger when one of them is charged. See here and here for a review of atmospheric nucleation processes.
Instead of nucleating into a new particle, H2SO4 could also condense on an existing aerosol particle, making it grow in size. Because of this competition for the vapor, nucleation is more likely to happen when there is only a little aerosol present.
Aerosol growth
Condensation of more vapor onto the nucleated aerosol makes it grow in size. However, other processes hamper its possibility to grow large enough to substantially influence the climate: Two aerosols can collide together, in a process called coagulation. Coagulation is particularly efficient between very small nano-particles and larger particles (of a few hundred nanometers). It causes the bigger one to grow in size, whereas the smaller (recently nucleated) one disappears. When there are a lot of very small aerosols around (i.e. after a nucleation event), they can also coagulate together. This causes them to grow in size, but decreases their number concentration. The loss processes for the number of aerosols (deposition and coagulation with bigger particles) are stronger when they’re very small.
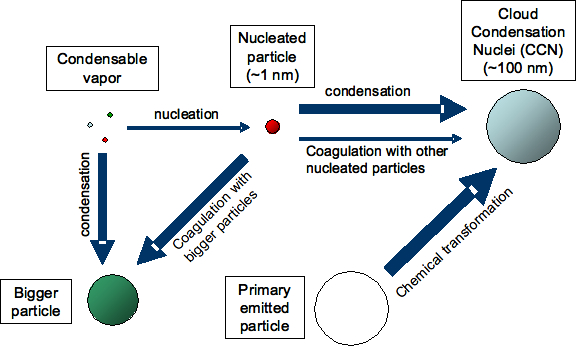
Figure 1: Different factors influence the extent to which nucleation contributes to the number of cloud condensation nuclei (CCN). (Figure partly based on AGU presentation by Jeff Pierce)
Measurements
New particle formation has been observed all over the globe, from the Poles to the Tropics, from urban to remote areas, and from surface sites to the upper troposphere (see here for a review of such observations). Of these locations, only nucleation in the free troposphere and in the vicinity of clouds seems to agree with theoretical predictions. In most other cases the number of aerosol particles produced is under-predicted. This has led to the development of semi-empirical approaches to describe nucleation. Laboratory studies have typically found much stronger dependencies on H2SO4 than atmospheric measurements. A confounding factor is that newly formed particles of 1 to 2 nanometers can not be directly measured by commercially available instrumentation (though there are new developments in this area). Nucleation takes place in a kind of no-man’s land between the gas and the liquid phase, about which we know surprisingly little.
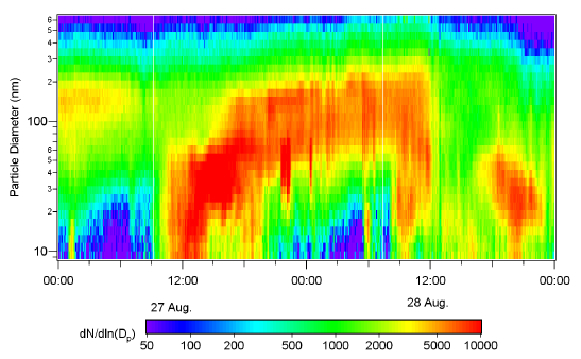 Figure 2: Measurements of an atmospheric nucleation and growth event in the Lower Fraser Valley, Canada. The color gives the (normalized) number concentration, where the red color indicates the enhanced concentration of nucleated particles, growing into the CCN size range. (from Mozurkewich et al.)
Figure 2: Measurements of an atmospheric nucleation and growth event in the Lower Fraser Valley, Canada. The color gives the (normalized) number concentration, where the red color indicates the enhanced concentration of nucleated particles, growing into the CCN size range. (from Mozurkewich et al.)
So what is needed for nucleation to occur? Favorable conditions include a strong source of condensable vapor; high UV radiation intensity; low aerosol surface area; high relative humidity; low temperature; presence of ions; and atmospheric mixing processes. Under different environmental conditions, different nucleation mechanisms may be at work. For example, in industrial plumes and over urban areas enough sulfuric acid may be present to form new particles and have them grow to a stable size. Ammonia may neutralize the acidic cluster, and thereby help stabilizing it. Over forested areas, the relative role of organic compounds is expected to be much larger (though a strong correlation of nucleation events with sulfuric acid remains). In coastal areas, iodine compounds are likely involved in the nucleation process. In the upper troposphere, the ion density is usually larger, whereas the sulfuric acid concentration is lower. The relative role of ion induced nucleation may therefore be larger up there. The dominant role of sulfuric acid has remained a steady conclusion over the years, whereas the potential roles of organic compounds and ions are still hotly debated.
In part II, I’ll discuss the potential importance of nucleation and of galactic cosmic rays for climate change.
Is this the topic under which to discuss Professor Salter’s proposals?
http://www.timesonline.co.uk/tol/news/environment/article4648680.ece
I have met Professor Salter, who has a history of being on the right side of the climate argument. He seems thoughtful and rational but when I talk to others who worry about climate change, I am told his proposals are too risky. In the current context should we not take him seriously – we are well past any safe option.
My gut feeling is that his is a route worth investigating. When I saw him a few months ago he was short of funding. Does his scheme deserve the money?
I’ve been wanting to ask you guys… aerosols are in the news at present, with the new paper just out on Arctic Warming. Shindell, Drew, and Faluvegi, Greg. Climate response to regional radiative forcing during the twentieth century, in Nature Geoscience 2, 294 – 300 (2009). Published online: 22 March 2009.
The authors, Drew Shindell in particular, are in the NASA climate modelling group. Shindell is an expert in atmospheric modeling, I gather. He’s written at least one article for realclimate in the past.
This new paper has attracted quite a lot of attention so far. The basic idea seems to be that the strong Arctic warming trend of 1.5 C/decade by comparison with a global trend of 0.2 C/decade indicates that there’s some regional effect which makes the difference from the rest of the planet. The authors single out aerosols, and black carbon, as the likely explanation.
Many of the usual suspects seem to be taking this paper as a sign that NASA has published a report indicating that changes in aerosols are responsible for global warming; rather than greenhouse gases. One report even speculates that Shindell’s position with his boss (James Hansen) has just become more difficult because of this.
[Response: Particularly amusing nonsense, since Jim has been pointing out the importance of black carbon deposition (which replaces the normally highly reflective snow surface with highly absorbing particulates, thus enhancing surface warming) on Arctic warming for a number of years. This effect is additive to the greenhouse gas warming. Any claim by the contrarian noise machine that this (very important) research by Drew and colleagues in any way challenges our understanding of the influence of rising greenhouse gas concentrations on the warming of the Earth’s surface suggests a remarkably deep level of confusion, disingenuousness, or both. -mike]
It’s certainly not how I saw the paper. But I’d love to see a clear comment on this from the authors.
[Response: Perhaps we can get Drew to stop by and comment. -mike]
What do you think about the US Administration’s opening to geo-engineering?
Since the second figure appears hard to read:
The vertical axis shows particle diameter (from 8 to 600 nm),
the horizontal axis shows time (two days in total),
color denotes number concentration, where blue is low, via green and yellow to red (=high).
On Geoengineering, my personal view is that it’s potentially dangerous, but so is unmitigated climate change. Those dangers should be compared, and more research is needed to do so. At this point in time, I would be against active deployment of geoengineering schemes, but in favor of more research into that area.
Duae Quartunciae #2, reminds me of a question that climate experts have told me that the answer is “No”. They are probably right but on these topics it doesn’t hurt to be sure. The question is
I have read that
1. Weather over Siberia can have blocking patterns with little wind.
2. Tamino’s blog, if I remember correctly, mentioned that rises in methane levels can vary by several weeks at different measuring stations.
3. Concentrations of methane near the emitting sources can be hundreds of times greater than background levels. Clearly, if these concentrations reached any height there would be a local warming effect. If these concentrations reached one hundred metres above the emitting sources these concentrations would occupy about one percent of the atmosphere above the source. One hundred times a background level would double the warming effect of methane in the locality.
Warming areas where methane is emitted with methane that is not well-mixed is clearly some sort of feedback. Is it vanishingly small? I would be interested to know if anyone has done the work to dismiss it definitively.
Perhaps it exposes my ignorance to say, but I found it surprising that “[a]lmost all of their properties […] are size dependent.” Does shape (flat or round) or density play no role in a particle’s tendency to remain airborne? I would think that, what ever effects a particle might have, the need for it to be airborne determines how much of the effect will be produced.
“Many of the usual suspects seem to be taking this paper as a sign that NASA has published a report indicating that changes in aerosols are responsible for global warming; rather than greenhouse gases. One report even speculates that Shindell’s position with his boss (James Hansen) has just become more difficult because of this.”
Ah! Yet another result from denialosphere creative writing machine. I just wonder, are there any constraints on what these guys can write. I mean it seems they are unconstrained by science, history, ethics… Can they construct a lie so bald-faced that even they coundn’t deliver it with a straight face?
Nice post, but how does that compare to aerosol formation during the process of fossil fuel combustion (especially diesel and ship bunker fuel)? Also, how is aerosol formation affected over cities by high level of ozone and peroxyacetyl nitrate (PAN)?
The relative contribution of fossil fuel combustion and biomass combustion to the global black carbon inventory is a matter of some dispute, which is also probably being affected by drought-correlated rise in global wildfires. This can also be seen in the rising rate of tree death across the world:
http://www.sciencedaily.com/releases/2009/01/090122141222.htm
However, the “global black carbon inventory” is a bit misleading, because aerosols are often the most short-lived component of the atmosphere (which is why regions near dirty coal plants are more soot-blackened than distant regions). This high level of atmospheric variability means that sampling at one or two sites and then project that to the global level is a hopeless exercise – quite unlike the case with CO2, which tends to have a 100-year half-life in the atmosphere. Even with CO2, to avoid the effects of local trends you have to go to places like Mauna Loa and Antarctica to get reliable global-scale measurements. If you like, you can read a discussion of the relative inputs to aerosol clouds here. You could also ask Andrew Revkin to withdraw his still-standing claim that most of the aerosols in atmospheric brown clouds are due to biomass burning.
There are also some important chemical differences in aerosols produced by diesel and ship bunker fuels, especially in combination with ozone, PAN and other air pollutants, for example:
http://www.ncbi.nlm.nih.gov/pubmed/18970748
While it is not the most pleasant topic, you can see what exposure to diesel aerosols does to mice:
http://www.informapharmascience.com/doi/abs/10.1080/08958370802112922
That’s not exactly, climate, no. However, the aerosol composition also has an effect on precipitation – see this 2002 article in Science on the effects in China and India:
http://irina.eas.gatech.edu/EAS_spring2006/Menon2002.pdf
Notice that the effect there is the opposite of the effect of adding volcanic aerosols, which cool the global climate due to stratospheric effects. Removing black carbon aerosols from China and India should have a cooling effect.
“Perhaps it exposes my ignorance to say, but I found it surprising that “[a]lmost all of their properties […] are size dependent.”” – jmh
That does not imply that they are not also dependent on other factors, such as shape.
What do you think of this story, dated April 10, 2009 http://www.dailymail.co.uk/sciencetech/article-1169007/Climate-change-goal-Laws-combat-acid-rain-DRIVING-Arctic-warming-claims-Nasa.html
The author contends, based on a recent NASA study by Drew Shindell of the NASA Goddard Institute for Space Studies in New York, that reduction of particulate emissions is hurting efforts to reduce global warming from CO2 — an own goal by the Green Team.
The warming at the South Pole (where aerosols are sparse) since the ’70s when scrubbing regulations began is 0.35C, which less than the 1.5C warming at the North Pole (where they are greater). And black carbon absorbs energy (like ozone) and aggravates global warming. However, there is also the global cooling effect of aerosols (such as in nuclear winter). So there seems to be some confusion in public opinion.
See the response to #2, Wilmot.
Stop cutting and pasting to “spread the word”. When you do that, it is far too easy to be outed as a non-sentient lifeform.
Mark —
Having read the response to #2 I respectfully reiterate my wish to point out the confusion in public opinion over the effect of aerosols. Abashed though I am at your rebuke, perhaps my contemptible efforts may serve as a guide to you and other sentient life forms to make a useful contribution.
Can somebody advise; would it be a better contribution to the climate problem to coat my dark shingled roof reflective white, or to park my Lesabre and get a bicycle? 10K mpy,20mpg, 3,200sf, WA.
The statement “… reduction of particulate emissions is hurting efforts to reduce global warming from CO2…” reflects a widespread and fundamental misunderstanding of the role of higher CO2 in forcing global warming. The increased CO2 will behave exactly the same regardless of the levels of aerosols in the atmosphere, changing the energy balance and warming the planet. Aerosols will also change the energy balance, and depending on the characteristics and circumstances, and secondary effects like influence on clouds, can heat or cool the planet.
Saying “reduction of particulate emissions is hurting efforts to reduce global warming from CO2” is nonsense (or spin from the coal lobby) equivalent to saying “turning off my air conditioner is hurting my efforts to keep my house cool by opening the doors and windows to let in cool breezes.”
Mark, if you look at Wilmot’s website, you’ll find …. um … no, don’t go there.
[Response: indeed. I’ve scrubbed the link. – gavin]
I think the Shindell paper (april issue Nature Geosciences) and the attempts to respin it by the fossil fuel lobby together demonstrate how media opinion is far behind the most recent scientific research into climate.
http://www.eurekalert.org/pub_releases/2009-04/nsfc-amd040809.php
So, in the scientific world, global warming is an accepted reality, backed up by observations and models – and the questions now are focusing on discrepancies between models and observations. The most obvious one is in the Arctic, where rates of sea ice decline are bypassing the model predictions by a good margin.
This discrepancy is the kind of thing that brings scientific attention – that’s how the model/observation approach works – it’s when the model fails that you learn something new. That’s the reason why the paper was considered significant.
So, if we make up a list of possible reasons for the model’s failure to predict Arctic intensification, we can include:
1) aerosol effects
2) dynamic increases in poleward heat transport
3) sensitivity of thin sea ice to wind forcing
The Shindell & Faluvegi paper seems to indicate that aerosols are the largest unaccounted factor in the climate models. Their abstract begins:
Those three correlate with the three factors I listed, sort of. There may also be additive effects, i.e. natural variability imposed on polewards heat transfer and sea ice (the Artic Oscillation, say).
In any case, it is simply an effort to reconcile the rapid rates of warming in the Arctic with the output of the most recent group of global climate models – everyone agrees that global warming is real, except for a very large number of editors and reporters with the U.S. press, who continue to advocate for the positions held by a small number of fossil fuel funded contrarians and insist on giving them “equal time” – a luxury denied to renewable energy experts. In the area of climate, scientists and denialists get equal time – but in the area of energy, the only side that you hear from is the fossil fuel lobby and their front groups.
The denialist intentional confusion between CO2 and aerosols is an example of why ALL college students, regardless of major, should be required to take the “Engineering and Science Core Curriculum.” Even drama, music and English majors should be required to take Engineering and Science Core Curriculum so that those who wind up doing a lot of writing will have some contact with reality. As it is now, they see everything as a word game. They need to be shown that NATURE is the boss, not the person with the fanciest rhetoric.
#17 Edward
I just asked my 9 and 11 year old sons whether the natural world controls our environment or someone who tells a good story. Both were quite capable of selecting mother nature as the boss. I doubt it is necessary to dictate course selections to college students.
Thanks
William
The Shindell abstract reads:
“Our reconstructions broadly agree with historical emissions estimates, and can explain the differences between observed changes in Arctic temperatures and expectations from non-aerosol forcings plus unforced variability.”
So observations are a bit more in line with expectations.
Off-topic a bit…Hargreaves & Annan have recently published a comment on a study by Chylek & Lohmann, which suggested lower climate sensitivity. The authors show that Chylek & Lohmann selectively used a few unrepresentative outlier data points to gain the lower estimate. They show that by selecting unrepresentative data and using the same methodology, they can also estimate higher levels of climate sensitivity than the 3 C IPCC best estimate.
http://www.clim-past.net/5/143/2009/cp-5-143-2009.pdf
I would also like to point out that the Daily Mail story (own goal by the Green Team) was featured on Environmental News Network, usually an advocate for clean tech. http://www.enn.com/sci-tech/spotlight/39648
An interview with Drew Shindell:
http://www.nytimes.com/2007/02/18/magazine/18WWLNQ4.t.html?_r=1&ref=magazine
I thought Dr. Hansen was all over the aerosols issue.
http://earthobservatory.nasa.gov/Features/GISSTemperature/giss_temperature4.php
and has been for some time. I thought they were supposed to be insignificant in that face of the the massive GHG forcings occurring. Are aerosols now going to dominate for a little while?
Geoff Beacon (1) — I recommend a e-mail to John Holdren at the White House.
Re The response on comment #2 by Mike:
“…. the importance of black carbon deposition (which replaces the normally highly reflective snow surface with highly absorbing particulates, thus enhancing surface warming) on Arctic warming for a number of years. This effect is additive to the greenhouse gas warming.”
This is pretty straightforward, and easy to understand by non atmospheric scientists,like myself. The albedo or reflectivity of the ice surface is decreased by coating the surface with black particulates which don’t reflect but absorb the heat from the Sun.
No rocket science here(though this a misnomer). Anyone who reads more than this into the subject is probably up to some kind of mischief.
William @18: Well, what can we say about someone who gets his science advice from 9 and 11 year olds.
@18
William says:
“just asked my 9 and 11 year old sons whether the natural world controls our environment or someone who tells a good story. Both were quite capable of selecting mother nature as the boss. I doubt it is necessary to dictate course selections to college students.”
Aerosols _are_ part of the natural world. It’s just they have been moved from one place or state to a different one.
CO2 is part of the natural world, as above.
Everything is part of the natural world.
I find the article on aerosols informative and understandable by not-too-scientific minds like myself. I have book marked it
This is a most excellent summary of atmospheric aerosol nucleation! Thorough, yet concise.
Jhm (6):
“Does shape (flat or round) or density play no role in a particle’s tendency to remain airborne?” Yes, but their effect is minor compared to that of particle diameter.
The effect of particle shape is sometimes included by multiplying the size by a ‘shape factor’ to arrive at an “effective diameter”: the diameter the particle behaves as. For atmospheric aerosol, this shape factor is usually not strongly different from one; its effect is usually assumed negligible compared to the effect of particle size, which covers several orders of magnitude. An exception is e.g freshly emitted soot, which is far from spherical (see some pictures of archetypical aerosol types here; soot = Russpartikel). Upon chemical transformation in the atmosphere, most aerosols become more or less spherical.
As for particle density, that also has a limited range (factor of two), so its effect is also very minor compared to that of particle size. Besides, everything depends on size, but not everything depends on density. Settling by gravitation depends on mass and thus on density, but that’s a relatively minor loss process at least for submicron aerosol. And in that case the diameter is having its effect raised to the cubed power.
Ike Solem (8):
I don’t know the specifics, but eg in the exhaust stream of cars the high supersaturation of organic compounds causes continuous nucleation, and indeed the exhaust pipes emit a lot of freshly nucleated nanoparticles of typically around 10-20 nanometers in diameter. In powerplant plumes or shiptracks nucleation can occur because of elevated levels of SO2 (which forms H2SO4 after photo-oxidation). High ozone levels downstream of pollution sources can cause elevated OH radical concentrations, which in turn increases the concentration of condensable species and thus the potential for nucleation. But the aerosol surface area is usually larger as well, and that would suppress nucleation. It’s not immediately obvious which process would win, but its effect would be extremely hard to detect regardless.
Good point about the difference in variability between aerosol and GHG.
Krog (13):
From a climate perspective, it’s much better to leave your car standing and ride a bike than paint your roof white.
Re: #1.
The suggestion of spraying sea salt particles into the air is an example of adding primary aerosol particles to the atmosphere. This is different from nucleation, which generates new /secondary/ aerosols via gas-to-particle conversion.
The idea behind spraying sea salt into the atmosphere is to increase the concentration of cloud condensation nuclei (CCN), which should increase cloud brightness (albedo) via the Twomey effect (and possibly increase cloud lifetime).
Mike,
Of course the effect of black carbon is additive. The effect would be to increase the Arctic temperature from what would have been caused by greenhouse gasses by themselves. This has the opposite effect of “white” aerosols which were damping the temperature increase.
[Response: Yes, that’s the point. Thanks for restating it. This seemingly obvious point is apparently lost on those who argue its an “either/or” proposition (i.e. the fatuous implication that somehow the research in question suggests that greenhouse gases are not primarily responsible for the observed warming of the globe). -mike]
To what extent do you believe black carbon has increased the measured Arctic and global temperature trends?
[Response: Firstly, its not a matter of “belief”, let alone what I believe. There is a solid literature on all of this, and there are entire chapters on this in the IPCC reports. The bottom line is that reflecting aerosols, at least, thusfar, have dominated the global mean anthropogenic aerosol forcing. That means that anthropogenic aerosols have been a mitigating factor when it comes to the warming of the globe over the past century. There is nothing in this latest study that challenges that. For the Arctic, its an entirely different story. Black carbon has likely played a far more significant role there, because of the contrast between absorbing aerosols deposited on the ice surface, and the high albedo perennial ice cover, and thus in this particular region black carbon has likely been an aggravating factor when it comes to anthropogenic surface warming. I apologize for leaving all of this implicit in my brief, original comments. For further details, see the IPCC AR4 Working Group I report (see in particular sections 2.4 and 2.5 of chapter 2). -mike]
Thank you for providing this very well written and easy to understand synopsis Bart. Very helpful, especially for ignoramuses such as myself.
Re: #1,
Imagine a 50 million dollar way to manufacture enough cooling clouds to offset AGW for as long as it takes to get emissions under control.
I’d say, even if that idea has only 10% merit today, it’s well worth taking seriously and exploring.
These are some of the concepts we are going to need to explore as we go forward. I’ve seen one or two of you stating as a given that emissions reduction will not be enough.
But I have no intention of hijacking three posts in a row, and I will decline to pursue that thought any further in this thread.
Let me just say the idea expresses a strong sense of rationality (working with nature the way nature works) and realism (we’re going to need to do more than we’re doing). On that basis alone it deserves notice.
would like to know how much aerosols were produced during WW2, the Korean and Vietnam Wars? If one looks at the global temperature variations during these periods one observes a declining temperature trend. Could there be a cause and effect relationship? I have been alife during that time and observed the enormous dstruction during these wars. For ore details review the monograph of Enrico Fabrizius \A Painful Reality\. Thanks for responding. Heinrich Schmid.
Ray #25:
> William @18: Well, what can we say about someone who gets his science advice
> from 9 and 11 year olds.
More to the point, those kids tend to have their curiosity intact. Something many manage to lose as they “grow up”. Is it too far out to suspect, like Edward Greisch does, that the education system has something to do with that?
Very well written article Bart, thanks for the effort. James Dorsey much appreciates the links to the introduction to his first year report from his Ph.D. Look forward to part 2.
David Benson #23. Thanks I did.
I had this tome foisted on me. I’d put this in the mostly natural category. To discuss.
“Melankovitch cycles also influence warming, not just cooling, and we are in a warming period of the cycles. As I believe I said earlier, but of course why let little things like that stand in the way of your continual pseudo-intellectual efforts to build your ego up. But, I guess, what can one expect from someone of the bent who judges scientific research on the basis of one’s own perceived political interpretations and labels it all knowing or conservative denying in turn.
But if we want to talk about things that man does impact greatly, CO2 releases would be one of them. However, the assumption invariably exhibited that industrial releases by us is the greatest cause of this is so incorrect it is laughable. The simple act of some civilizations trying to feed and clothe their members through subsistence existences is by far a larger source to the extent that the United States could stop all emissions this very second along with the industrialized world and CO2 levels would still be projected to increase by a factor of 3 by 2100. Deforestation from subsistence groups, primarily in Africa and South America, produces 30% of the total global CO2 release while industrial sources contribute 14%.”
[Response: Very wrong. Last figures available show about 7 GtC/yr from industrial (fossil fuel/cement) and ~2 GtC from deforestation. – gavin]
“I’d put this in the mostly natural category.” – Mark A. York
I’d say the rubbish you received also has a large component of “blame it on the poor” (a.k.a. “blame it on the dark-skinned”). The writer’s use of “industrial sources” as a comparator with deforestation is either ignorant or disingenuous: most of the industrialised world’s CO2 production is from domestic use, travel, land use change and other “non-industrial” activities. Moreover, a large proportion of deforestation is not taking place for subsistence agriculture, but for cattle ranching, oil palm plantations, timber extraction and other commercial enterprises – many of which are supplying raw materials to the rich world.
Hi Tom (35) and others above, Thanks for your positive feedback. I mistakenly doubled up on the link though.
Thanks Gavin. Good point Nick.
“Aerosols are liquid or solid particles suspended in the atmosphere (but not including water droplets or ice crystals)”
I think this is likely to be misinterpreted. By mass aren’t most aerosols in the atmosphere mostly water? While water may not be significant in the formation of the aerosols it can play a big role in the growth and evolution of particles.
> mass … mostly water?
Maybe residence time affects the population?
The first link in the first post mentions that some accumulate water, and so wash out as rain.
Poking around with Scholar, I found mention of “hydrophobic soot particles from residential coal and industrial oil burning” and also mention of radar being used that distinguishes aerosols from water vapor and clouds.
Re #37: “Melankovitch cycles also influence warming, not just cooling, and we are in a warming period of the cycles.”
Noone has yet commented on this sentence from the person who you are corresponding with, so I’ll take a crack.
First of all, I wonder where he is getting the idea that we are in a warming period of the cycles. The long term trend is actually expected to be toward cooling, being that we are in an interglacial period. I guess it was originally expected that “long term” meant over say the next 20,000 years (as Shackleton et al talked about in their classic 1976 paper in Science)…although some of the latest work predicts this interglacial period would (even in the absence of human interference) be expected to last longer, as I recall perhaps another 50,000 years. Now, it may be that along with this longer interglacial comes the idea that over shorter timescales (of, say, 10000 or 20000 years) we would actually expect some a little warming although I haven’t heard this case made…and would expect that any such effect would be quite modest.
Second of all, Milankovitch cycles operate on much longer timescales than the timescale of a century or so that are of interest to us at the moment. Even the warmings out of the glacial periods into the interglacials only occurred at an average rate of something like 0.1 C per century (with coolings generally being even slower). So, even if your correspondent were correct in his claims about where we are in the cycles, I don’t think it could account for very much of the warming.
Nosmo (41) and Hank Roberts (42):
Water droplets and ice crystals are usually treated separately from aerosols because of their different nature. However, the distinction is not always crystal-clear.
Most regular aerosol particles contain water, the amount of which depends on the hygroscopicity (=water affinity) of the aerosol and on the relative humidity (RH). At 95% RH, water is perhaps the dominant compound in most aerosol types, but at say 50% RH, that’s not the case. The phenomenon of haze limiting visibiliy is often aggravated by a high RH, causing the aerosols to grow in size as a result of picking up more water. Since they’re bigger, they scatter radiation more effectively, hence the aggravating effect on visibility.
Freshly emitted soot is an example of a hydrophobic aerosol type; after chemical transformation in the atmosphere (e.g. oxidation of its surface rendering it more hygroscopic; condensation of more hydrophilic species onto the particle) it will gradually pick up water as well.
Radar distinguishes cloud droplets (~10 micrometer) from aerosol particles (number dominated by those
Since it’s been known for some time that black carbon is a significant cause of Arctic warming, what is the reason that it and other aerosols have not been factored into the climate models —for what reason would such an important factor be left out?
It seems especially important in the light of the research and findings by Lehmann et al on black carbon in soils—- its very slow decomposition rate—–and the fact that climate models may be over-estimating their global warming predictions, if they are not including ‘realistic stocks of black carbon in prediction models’.
http://climateresearchnews.com/2008/11/study-on-soil-black-carbon-suggests-global-warming-overestimated-by-climate-models/
[Response: Why do you think they are not included? They are. – gavin]
Re: Truth (#45): Speaking of the Lehmann et al. study, I thought that it might be nice to have realclimate do a response to it. I did send a note to the Cornell press office along these lines:
“I read the recent report of Professor Lehman’s study on black carbon in Australian soils with interest. However, I might suggest that the implications of the study for global climate projections have been overstated. The positive feedback of increased soil temperature leading to increased decomposition and therefore natural carbon emissions is a fairly modest contributor to the total projected business as usual carbon emissions over the century: average IPCC AR4 model land carbon storage changes due to climate change yielded a 63 ppm CO2 increase over the counterfactual by the year 2100. Therefore, an assumption that the 20% reduction in land carbon storage resulting from the Lehman et al. work holds globally would yield a reduction on the order of 13 ppm. 13 ppm, while not negligible, and certainly of significant scientific interest, is still small in comparison to the several hundred ppm increase expected in the business as usual case.
Therefore, I would suggest that the title of the Cornell news release “Global warming predictions are overestimated, suggests study on black carbon”, while technically correct, is rather misleading in that a casual reading would infer an “overestimate” rather larger than the couple percent implied by the study.And, indeed, the news release in being cited in a number of places on the internet as evidence climate change fears are being overstated.
If I misread the study I apologize, but I do believe that a rewording of the news release would reduce the potential for misinterpretation, and I thank you for your attention,”
I got a polite, agreeable, but somewhat non-committal response to my comment from Prof. Lehmann. Truth, you might also want to note that “soil black carbon” is a completely different beast from the aerosol black carbon issue.
Here, from the lead paragraph of the most recent post, is all you need to know about the source from which “truth” obtains his (her, their?) ‘truths’:
New Scientist, also known as Nude Socia.list [spam block avoider there] magazine, never misses the opportunity to use the derogatory phrase ‘climate-change deniers’ in order to smear sound scientific argument against an unverifiable computer modelled catastrophe driven by harmless aerial plant food gas.
‘Nude Socia.list’, oh man that is too funny? I spit coffee all over the desk when I read that. Actually all kinds of funny stuff over there. That guy must write for Letterman.
“Water droplets and ice crystals are usually treated separately from aerosols because of their different nature. However, the distinction is not always crystal-clear.”
Without a condensation nuclei, water vapour won’t condense unless supersaturated (130%?) or VERY cold (
Jim, that’s a veritable workshop on how to pack the most loaded modifiers into one sentence–albeit at the cost of redundancy and vagueness. (If there’s “aerial plant food gas” can there be “terrestrial plant food gas?” And does aerial modify “plant” or “gas?” EB White would not approve.)
(Captcha disapproves, too: “final-exam rented” and then “copying securely”)
Ironic “truth”:
“Since it’s been known for some time that black carbon is a significant cause of Arctic warming, what is the reason that it and other aerosols have not been factored into the climate models —for what reason would such an important factor be left out?”
It’s ok, you can come out and say what you’re thinking… It’s a conspiracy! For a pittance of free conference travel, the possibility of an upgraded office with south-facing windows plus a few other perks the entire scientific community (apparently starting as far back as the mid-19th century) has constructed a nefarious and intricate plot, caring not a whit about their drastic and unfair impact on everybody else! Horrific, but then life is hardly fair– just ask ExxonMobil’s board of directors about the suffering they’ve been incurring at the hands of the malefactors.
My suggestion is that you resign as footsoldier for the beleaguered, temporarily and only coincidentally rich-as-Croesus industrial victims you’re so bravely defending, join the conspiracy, get in on the free coach class and peanuts. If you can’t bring yourself to sign on for all the largesse you’ll receive (free Internet with classy “.edu” TLD, etc.), watch out for scientists with white Persian cats, dueling scars and weird middle European accents; ff you can turn around fast enough you just might see them huddling together as they perform the difficult task of falsifying diverse observations from disparate fields in a way that consistently supports their conspiracy (an effort some might say is not worth it compared to earning billions as an industrialist, but the human nature is –so– complicated).