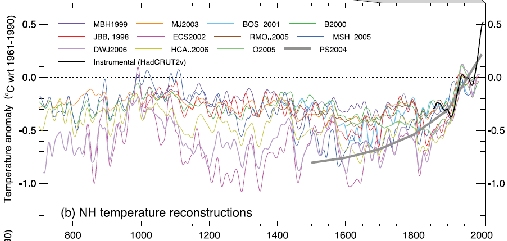
Much research effort over the past years has gone into reconstructing the temperature history of the last millennium and beyond. The new IPCC report compiles a dozen reconstructions for the temperature of the Northern Hemisphere (including of course the original “hockey stick” reconstruction, despite opposite claims by the Wall Street Journal). Lack of data does not permit robust reconstructions for the Southern Hemisphere. Without exception, the reconstructions show that Northern Hemisphere temperatures are now higher than at any time during the past 1,000 years (Figure 1), confirming and strengthening the conclusions drawn in the previous IPCC report of 2001.
Fig. 1: Figure 6.10 (panel b) from the paleoclimate chapter of the current IPCC report (see there for details).
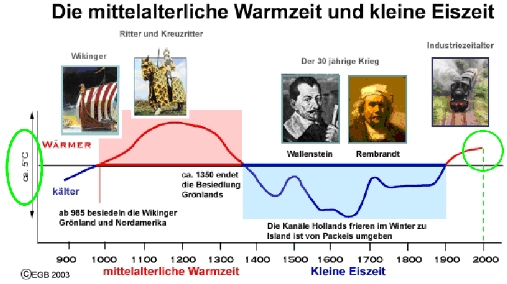
“Climate sceptics” do not like this and keep coming up with their own temperature histories. One of the weirdest has been circulated for years by German high-school teacher E.G. Beck (notorious for his equally weird CO2 curve). This history shows a medieval warm phase that is warmer than current climate by more than 1 ºC (see Figure 2). So how did Beck get this curve?
Fig. 2, modified from E.G. Beck (we added the green parts).
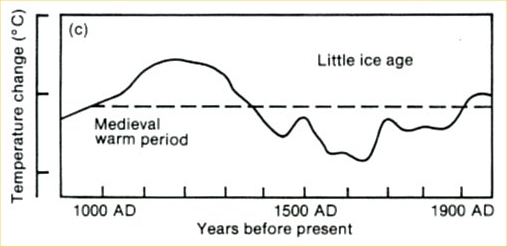
The curve is a fake in several respects. It originally is taken from the first IPCC report of 1990: a scan of the original is shown in Figure 3. At that time, no large-scale temperature reconstructions were available yet. To give an indication of past climate variability, the report showed Lamb’s Central England estimate. (Unfortunately this was not stated in the report – an oversight which shows that IPCC review procedures in the early days were not what they are now. We will post in more detail on the history of this curve another time.)
Fig. 3. The past millennium as shown in the first IPCC report of 1990, before quantitative large-scale reconstructions were available. This curve was based on Lamb’s estimated climate history for central England.
But Beck did not stop at simply using this outdated curve, he modified it as highlighted in green in Figure 2. First, he added a wrong temperature scale – the tick marks in the old IPCC report represent 1 ºC, so Beck’s claimed range of 5 ºC exaggerates the past temperature variations by more than a factor of three. Second, the original curve only goes up to the 1970’s. Since then, Northern Hemisphere temperatures have increased by about 0.6 ºC and those in central England even more – so whatever you take this curve for, if it were continued to present, the current temperature would be above the Medieval level, as in the proper reconstructions available today. As this would destroy his message, Beck applied another fakery: he extended the curve flat up to the year 2000, thereby denying the measured warming since the 1970s. With this trick, his curve looks as if it was warmer in Medieval times than now.
When approached directly about these issues, Beck published a modified curve on a website. He changed the temperature range from 5 ºC to 4.5 ºC – but he shortened the arrow as well, so this was just cosmetics. He also added instrumental temperatures for the 20th Century at the end – but with his wrong temperature scale, they are completely out of proportion. (In fact his version suggests temperatures have warmed by 2 ºC since 1900, more than twice of what is actually observed!)
Beck goes even further: in a recent article (in German), he has the audacity to claim that his manipulated curve is right and the more recent scientific results shown by IPCC are wrong. And for years, he has offered his curve on an internet site (biokurs.de) that distributes teaching materials for schools, with support from German school authorities. It is quite likely that his fake curve has been shown (and will continue to be shown) to many school children.
499 BPL
Molecules can variously absorb and emit, but if you want a molecule to capture heat, which is what we need for a GHG, it needs to be absorbing and re-radiating, not locked up in some excited state. You can do this by whacking it with another molecule for a non-radiative transition, or cross couple it to molecules of lower energy. Otherwise it radiates at a frequency at which many of the remaining molecules are white, thereby losing energy outer space.
While I generally like the curves in the section
Part II: What Angstrom didn’t know
I note these molecules are much closer together and subject to collision broadening than in real life.
re: 329
The ultimate source —
http://cfa-www.harvard.edu/hitran//
actually not that difficult to use, just cumbersome.
Allan Ames (#500) wrote:
Different approximations for different levels of detail or different purposes (e.g., altitudes) I would presume.
I guess the question isn’t how reasonable the output might seem to one person or another, but how well they correspond to what we actually observe. From what I understand, clear sky is a fairly good match. Clouds were a problem in the 1990s, and the exaggerated thickness of clouds has continued to be a problem, but next generation Hadley does a much better job.
But perhaps you know better than I do. Probably not, though, at least judging from your questions.
Re 495
Allan, I am afraid I cannot agree with you regarding CO2 and CH4 being greenhouse gases. Venus is kept very warm by CO2 and very little water vapour. Moreover, I am quite happy to ignore the continuum effect of water vapour since, as Barton says, it has a very small absorption coefficient.
But I am very interested where you mention that Planck excluded fluorescence from his blackbody function B(T). Do you have a reference to the paper where he wrote that, because Einstein wrote the opposite in a paper that is translated in a book called “The Old Quantum Theory.” He claimed that gases do obey Kirchhoff’s Laws, which include radiating as a blackbody.
As I see it, the current models have two errors. First, the radiation from the surface which is absorbed goes to heat the air and so cannot be re-emitted. Secondly, there are emissions from the greenhouse gases which are caused by collisions, but they depend on pressure not temperature. In other words the greenhouse gases do not radiate according to Planck’s function, which depend on temperature.
Alastair: Thank you for the gentle question. I admit to having exaggerated somewhat, and have been surprised at the comparative lack of reaction. Essentially what I have done is to say that a “green house gas” is a gray body (if and only if). But beyond this manipulation of words there are some serious modeling issues. I believe the principles are as follows.
Quantum mechanics dictates that isolated molecules will have particular energy levels. Once launched, heat radiation is no different from any other radiation, and the rules for its absorption, etc, are the same as for any other radiation. Compared to visible light, 300K heat radiation is mostly in vibration-rotation rather then electronic. Two quantum oscillators will demonstrate increasing splitting as the coupling increases. Tightly coupled quantum systems act as a single entity. Partly coupled systems have mixed characteristics. From the quantum standpoint, I need to distinguish quantum oscillators and energy systems that are uncoupled like isolated gas molecules, partly coupled like water molecules, or fully coupled like solids or CO2 on Venus or dense planets. Intermolecular coupling of quantum states is the very important consideration.
From the Introduction; Planck’s “The Theory of Heat Radiation”, Dover Publications, 1959:
“Radiation of heat, however, is in itself independent of the temperature of the medium through which it passes. It is possible, for example, to concentrate the solar rays at a focus by passing them through a converging lens of ice.”
“We shall now introduce the further simplifying assumptions that the physical and chemical condition of the emitting substance depends on but a single variable, namely, on its absolute temperature, T. A necessary consequence of this is that the coefficient of emission ï�¥ï� ï� depends, apart from the frequency ï�®ï� and the nature of the medium, only on the temperature T. This last statement excludes from our consideration a number of radiation phenomena, such as fluorescence, phosphorescence, electrical and chemical luminosity, ——. We shall deal with “temperature radiation” exclusively.”
Just as a single copper atom does not make a conductor, a single IR absorbing molecule does not make a black body. Planck makes clear he is talking about bodies that are “black”, that is, characterized by a continuum absorption and radiation bands. I can dig out some references if you want, but the point is that thermal radiation is a property of materials with emissivities that do not vary (greatly) with wavelength. (My statement: d ï�¥ï� /d ï�® ~ 0 ).
It is clear that Planck is talking about systems consisting of tightly coupled radiators. And it is equally clear that your average gas is not a black body. Now let us ask the question, – Can gases be “gray” bodies? – and here I am moving into my own less well supported opinion. (The usual defintion of a “gray” body is one which partially reflects, like a cloud, but I am including partially transmitting bodies.)
The answer is – only if there is a continuum, or the lines broadened to overlap. And why is this? First, the continuum provides some coupling to the external radiation field, but mostly because the continuum signals that the vibrational energies are coupled, at least occasionally. Energy absorbed at one molecule is made available to other molecules, at least occasionally.
Raw broadening of molecular bands into system wide levels from proximity is what happens on Venus, sort of a “stochastic solid”. Schuster-Schwarzchild should work just fine on Venus.
Quantum coupling can be of many different flavors, depending on the transitions, but one of the easiest to understand is that a fluctuating dipole on one molecule can couple to another dipole, and water, unique with its ~1.5D dipole can couple across several, ~8, angstroms.
Things which can be “black” are soot and charcoal particles, metals (as fine particles), and CO2 on Venus because of concentration broadening.
Our problem with “white” gases is that the only mechanism for coming into equilibrium is collisions, and (I think I recall that) collisions need to occur at a rate roughly 10 times the Einstein A coefficient to hold equilibrium.
For any group of GHG’s acting like a “gray body” there will be an observable continuum.
To your comments – “As I see it, the current models have two errors. First, the radiation from the surface which is absorbed goes to heat the air and so cannot be re-emitted.” Yes. Particular CO2/Me frequencies are “removed” from the spectrum, and since the absorbers are decoupled from the other modes, there will be no subsequent repopulation. In the case of H2O, repopulation will take place to the extent of the continuum, and/or water line broadening , which will have somewhat different properties from the isolated vibration modes because of the coupling.
“Secondly, there are emissions from the greenhouse gases which are caused by collisions, but they depend on pressure not temperature. ” I think the collisions are not at all well coupled into the emissions.
“In other words the greenhouse gases do not radiate according to Planck’s function, which depend on temperature.” Absolutely. The two systems are effectively at different temperatures.
On writing this, it is time for me to take another look at the high-res IR upwelling data.
Looking forward to your evaluation.
“Often wrong, but never uncertain”
Allan Ames
Hi Allan,
I agree with most of what you say, but then you ARE agreeing with me :-)
I would say forget about gray bodies. They are not used any more. They were used to average bands but that is invalid. Just go with blackbodies. They are bad enough :-( They were invented by Kirchhoff for his law which is actually four laws; see Goody and Yung.
Two of these laws are (1) blackbodies radiate according to Planck’s function, and (2) in thermodynamic equilibrium emission equals absorption. This later statement is true for the surfaces of solids and liquids due to the Law of Conservation of Energy. If the system is in equilibrium the heat (radiation) in must equal the heat (radiation) out. But a blackbody radiates with Planck’s function whether it is in equilibrium or not. So if a gas obeys Kirchhoff’s Law does that mean it is radiating with Planck’s function or does it mean that its emission equals its absorption?
So a greenhouse gas does not radiate like a blackbody just because it is in thermodynamic equilibrium.
Anyway the argument has moved to here: https://www.realclimate.org/index.php/archives/2007/06/a-saturated-gassy-argument-part-ii/#comment-35977
where Ray Pierrehumbert is arguing that Einstein proved greenhouse gases do emit with Planck’s function. So either Planck or Einstein are wrong!
Cheers, Alastair.
For your information: Ernst Beck has published an article as a response to the uprising criticism here and by Urs Neu (ProClimate) on Readers Edition: Antwort auf “Klima-Kritik: Daten und Grafikmanipulation”.